Clarifications and 3 major changes include the updated National Adult Immunization Program for the period 2025-2026. In the new circular signed by the Deputy Minister of Health, Irini Agapidakis, there are targeted instructions for vaccines COVID-19RSV, HPV and influenza.
The aim is to give the necessary emphasis to the protection of the elderly and all vulnerable groups. The new circular recommends expanded protection through preventive vaccinations for more age groups, while simplifying the process of simultaneous administration of vaccines for COVID-19 and influenza and strengthens the protection of the elderly, pregnant and immunocompromised.
National Immunization Program: New recommendation for COVID-19 vaccine
The most important change concerns the vaccine against COVID-19, as well a second dose is introduced of the updated monovalent vaccine for people over 75 years of age, with an interval of 6 months.
In addition, it is specified that vaccination can be done at least 3 months after previous vaccination or illness, while for the first time co-administration with the flu vaccine is officially allowed, “on the same day, but on a different part of the body”.
This addition aligns the Greek program with the ECDC and WHO recommendations, which now treat COVID-19 as seasonal virus with booster doses in susceptible groups.
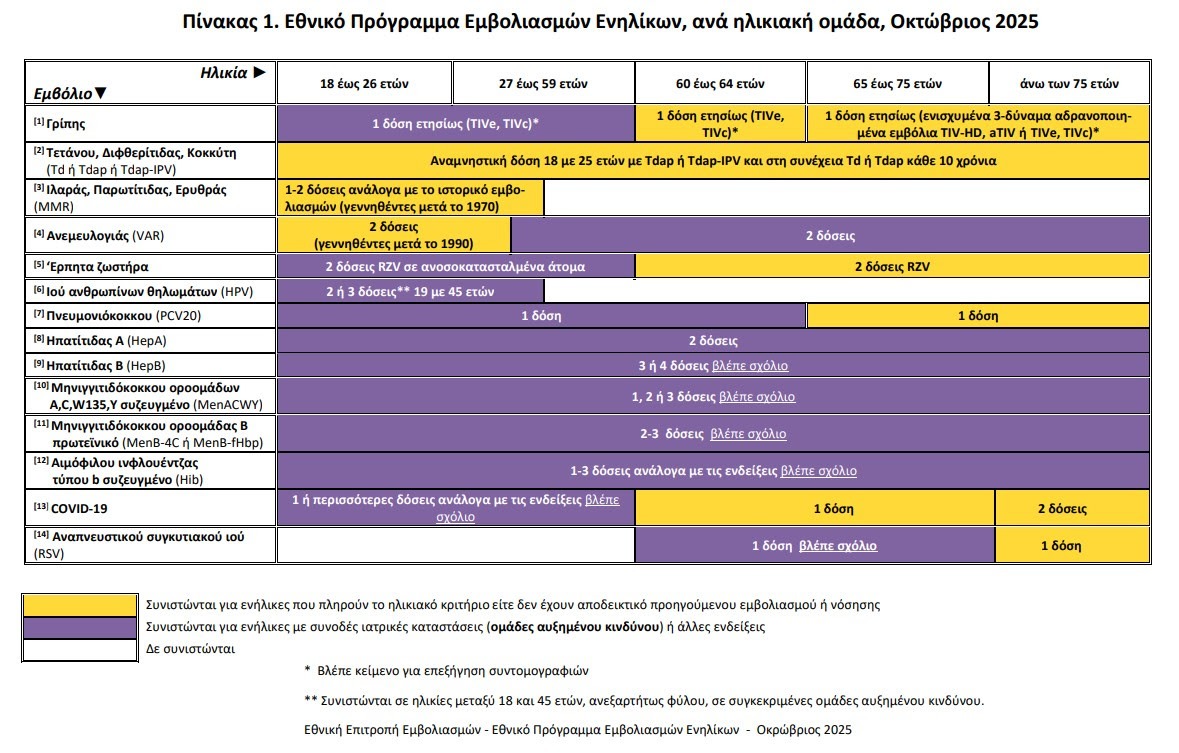
RSV vaccine: Expanded to more and pregnant women
For the first time, the circular expands the recommendation of vaccination against respiratory syncytial virus (RSV) in adults over 60 years of age with underlying diseases, and not only in people over 65 years of age as was the case until now.
At the same time, a recommendation for vaccination is introduced RSVpreF (Abrysvo), in pregnant women over the age of 18, between 32 and 36 weeks of pregnancywith an expected delivery date of November to March. This recommendation aims to protect newborn from severe respiratory infections during the winter months.
It is worth noting that was abolished the earlier warning to avoid co-administration of the vaccine RSV with hers flu or COVID-19.
HPV: Protection recommendation extended to more adults
The 2025 program includes extension of the age limit for human papillomavirus vaccine (HPV). Now, it is recommended not only for women but also for men up to 45 years oldas long as they belong to high-risk groups and have not been vaccinated.
It is preserved full compensation for 15 to 18 year olds until the end of 2026, while the program now explicitly refers to nine-valent HPV9 vaccine, covering nine strains of the virus.
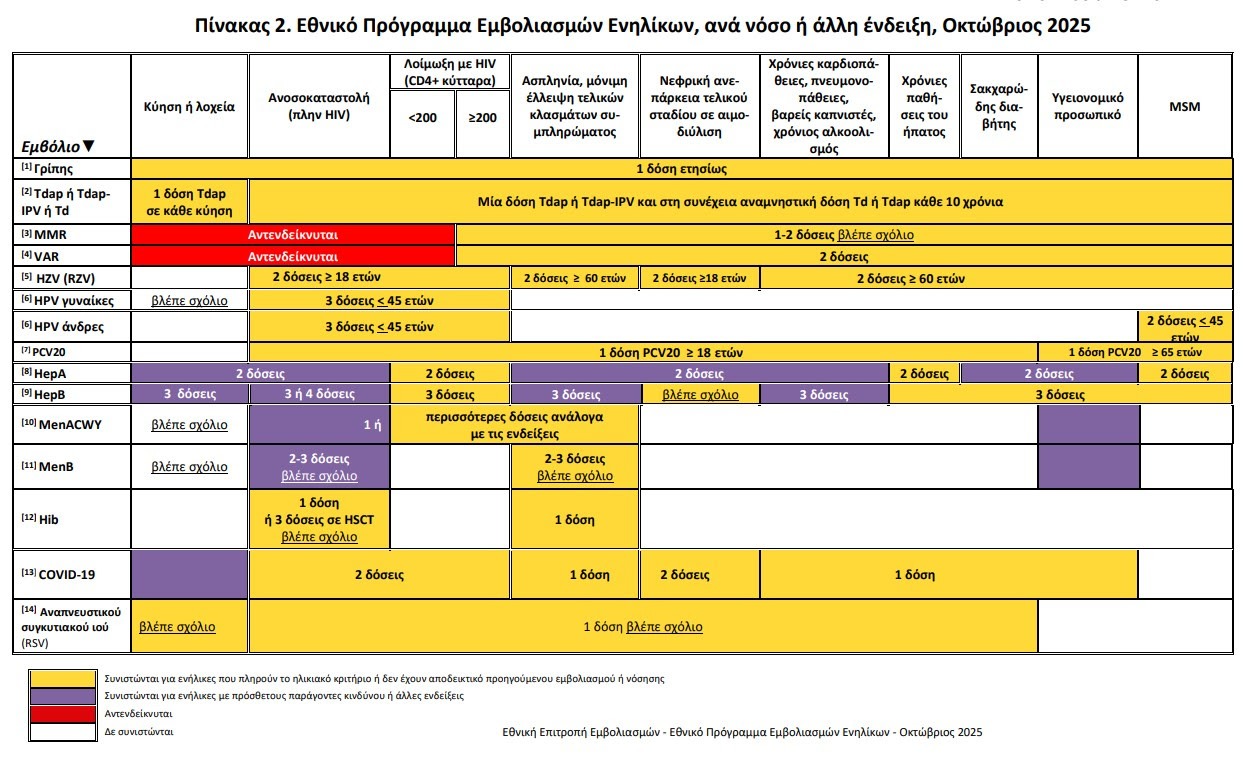

Flu vaccine: Priority for the elderly
Influenza guidelines emphasize the use of boosted vaccines in individuals over 65 years old, as well as in transplanted 18-64 years old.
In case limited availabilityvaccination priority is given to individuals over 75 years old.
The circular reminds that vaccines COVID-19 and flu can be done at the same timethus facilitating the planning of pharmacies and doctors.
Explicit reference is made to the use quadrivalent (QIVe) just in case depletion of stocks of trivalent vaccines.
Pneumococcal vaccine: Latest data on the new 20-potency (PCV20)
The new PCV20 vaccine (20 strength) remains the primary choice for pneumococcal disease prevention, but the new circular includes more detailed guidance.
In particular, it is suggested that those who have already vaccinated with PCV13 or PPSV23with time distance 1 or 5 years old respectively, from vaccination with PCV20.
The list was also updated high risk groups (patients with chronic heart disease, COPD, diabetes, kidney or liver failure).
Shingles vaccine
Vaccination with recombinant vaccine (RZV) is provided for:
- adults over 60 in the general population,
- and people over 18 years of age with immunosuppression.
The Commission, however, suggests that vaccination be preferred after 70 years for healthy adults, due to its effect immunoaging.
Other changes
Also included in the new program are revised tables, consistent with recent CDC guidelines (2024), for:
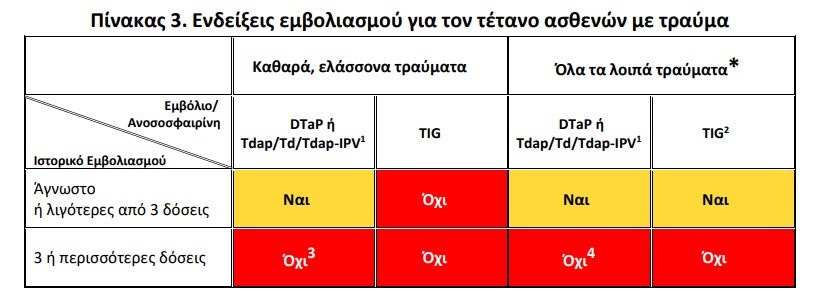
- tetanus and diphtheria in trauma
- vaccination transplanted patients

These changes reflect the shift of the program to a more “personalized” approach to vaccinationbased on each person’s age, underlying diseases and immune status.
For health professionals, the 2025-2026 program is – as stated – tool rational prioritization and better coordination of vaccination actions.